‘Pinna’ means feather in Latin, and yet it’s mammals, not birds, that have an ear pinna: that auricle projecting from each side of your head. Okay, so no bird has external ears like those of mammals, but is this a biological trivium or a real mind-teaser?
If the feathered members of the living fauna seem to proclaim ‘We birds simply don’t have external ears’, we may not be listening to the full symphony of facts sung to us by their anatomy and paleontology.
For there’s a noisy irony here. The feather is, after all, the definitive feature of living birds – the only feature possessed by all birds but no other clade of living animals.
It’s surprising that no[1] bird uses feathers to form paired auditory pinnae, for the following reasons. Certain owls have apparently suitable tufts of feathers nearly in place, and these tufts are individually mobile by muscular control, yet the birds seem determined not to use them to enhance hearing. Barn-owls[2] do use the plumage in the same part of the head to intensify sound, by ruffing[3] the whole facial rim of skin and short, stiff feathers into a kind of adjustable sonar disc. But this is no lookalike for paired external ears. Given that the large tubular eyes of owls are encased in their own bony skeleton and fixed immobile into the orbits of the skull, it seems almost perverse that owls haven’t claimed their birthright – pinnae – for listening. Instead they’ve compensated by having necks so flexible as to allow the face to swivel right around, plus a left-right asymmetry in the ears[4], something unknown in any mammal.

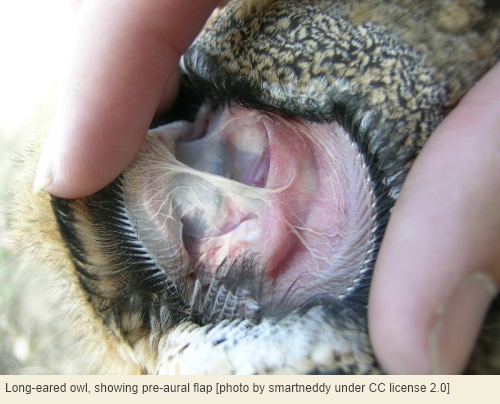
This failure of owls to evolve ear pinnae is even more surprising considering that certain species – particularly in the subfamily Striginae – do actually possess an auricle of sorts, which is called a pre-aural flap[5]. This is situated in front of, not behind, the ear opening, but can be surprisingly large and mobile. In the long-eared owl[6], the pre-aural flap is about 4 centimetres long and one centimetre wide, and when erected by muscles can potentially reflect sound waves coming from behind the owl’s head towards the ear opening. This is, in effect, a kind of back-to-front auricle, although admittedly small compared to the auricles of most like-size mammals and normally hidden by the facial plumage.
Of course, the difference between birds and mammals in the development of external ears could just be a phylogenetic accident. After all, not just birds but all living archosaurs lack paired ear pinnae, whether made of skin and cartilage as in the auricles of mammals, or made of skin and feathers as in the facial ruff of barn-owls. But things are not as simple as that. As we’ve just pointed out, certain birds do have comparable structures by a different name. And even crocodilians[7] have an arguable prototype for an external ear. So what would be required for the evolution of auricles or pinnae in birds is not invention but merely enlargement and modification. We can’t just assume that dinosaurs – the ancestors of birds – and pterosaurs all lacked ear pinnae, whether these are called auricles (made of skin and cartilage) or true pinnae (made of adjustable tufts of feathers).
We’ve mentioned the ‘ears’ of tufted owls[8] and eared owls[9], but an equally good example is the even longer tufts of so-called ‘pinnated grouse’, viz the prairie-chickens in the genus Tympanuchus. As in owls, there’s no evidence that these pinnae in grouse actually enhance hearing, despite their position close to the ear openings. And in the case of grouse, the erectile tufts are sexually dimorphic, making males in breeding plumage among the more bizarre examples of Darwin’s sexual selection. However, what grouse show is that there’s no phylogenetic impediment to birds having ‘external ears’ made of feathers, conforming to mammalian auricles in being mobile by muscular attachment. The feathery ‘ears’ of both owls and, by independent evolution, prairie-chickens can be erected or laid flat. This implies their potential – depending on slight relocation and reconfiguration – for use like mammalian ear pinnae to channel sound towards the ear opening.

Why, then, does no bird cock an ear?
Let’s go with two consistent avian themes, namely streamlining and the reduction of body mass far from the centre of gravity. With these in mind, a lack of auricles (i.e. ear pinnae made of skin and cartilage) would seem unremarkable in volant birds. However, a light version of the ear pinna, made of feathers, still seems like a viable option in some birds – remembering that certain terrestrial birds are flightless, and that the breeding plumage of many flying species includes handicaps such as elaborate appendages to the head. What’s particularly noteworthy is that the head adornments of birds include fleshy wattles as massive as the auricles of like-size mammals. And these flaps aren’t confined to males in breeding plumage. A cockerel may have no ears to cock, but the combs it does cock are as big as ears. Indeed, the fleshy wattle just below each ear openings of the domestic fowl[10] is actually called the ‘earlobe’ by fowl breeders.

What’s more, the typical avian constraints on mass seem to have been relaxed in birds such as kiwi[11], which carry the largest of all eggs proportional to the body and seek refuge underground rather than in the air. Why is it, then, that ground birds as aberrant as kiwi have not evolved ear pinnae? After all, kiwi resemble mammals in certain ways such as the placement of nostrils at the tip of the rostrum, and the profuse development of ‘whiskers’[12].
The fact that kiwi are also among the most extensive of burrowers among birds does not – by analogy with moles and mole-rats – necessarily explain their lack of ear pinnae because the head isn’t used for excavation. Although the beak of kiwi does probe the soil when the bird forages on the surface, it’s far too soft and sensitive to be wielded as an instrument to excavate refuge burrows; so kiwi ears don’t necessarily rub hard against the sides of the relatively wide tunnels dug by these birds. Kiwi also have surprisingly poor eyesight for nocturnal birds and compensate for this to some degree by using the touch-sensitive beak tip as a ‘white walking stick’ in conjunction with the ‘whiskers’. What this adds up to is an incongruous failure by kiwi to converge with mammals in the development of ear pinnae – if not from skin and cartilage then at least from the bristle-like feathers that these birds possess aplenty.

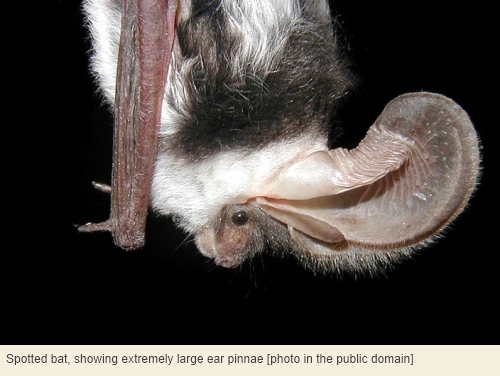
We can also go with another avian theme, namely that – despite the exception of kiwi – birds tend to have superior vision in compensation for inferior olfaction. However, there’s no reason to believe that, in general, birds depend less on hearing than mammals do. Birds are intensely vocal socially, and this includes nocturnal birds. Furthermore, oilbirds[13] use echolocation in much the same way as bats do. And bats are, in a sense, the opposite of birds despite sharing with them the constraints of body mass and streamlining that go with true flight: all bats possess auricles and some bats have extremely elaborate external ears of the fleshy kind.
Let’s summarise so far.
Considering the avian premia on sharp hearing and economical body mass, what we might have predicted in birds is the repeated evolution of light-weight ear pinnae made entirely out of feathers, which can be folded for streamlining in flight but then erected and appropriately rotated after landing. Although mere feathers may not seem substantial enough to reflect sound in the way a fleshy pinna does, owls once again seem suggestively perverse. Owls are well-known to muffle their flight by means of subtle adaptations in the structure of their flight feathers. If it’s possible to modify some feathers to absorb sound, it should be possible to modify other feathers to reflect sound instead.

So, we ask again, why does no bird cock an ear?
We do have a partial answer to the puzzle of the bird-mammal difference. Birds may use the skull itself, instead of external ears, to establish the direction of sound.

Unlike mammals, birds have both a complex system of air-filled auditory sinuses and an air passage connecting the middle ear on the left with the middle ear on the right. So the whole posterior part of the skull is a resonant system for sound, in which the sound waves continue to pass through a gaseous medium even after entering the skull.
Although some mammals also have auditory sinuses[14], these are not as complex or pervasive as in birds, and are not connected between left and right. And although living reptiles share the right-left connection of the middle ear with birds, their crania are not as hollow as those of birds. What this means is that, in the living fauna, birds have a unique degree of tympanic pneumaticity that connects left and right ears.
The distinctive anatomy of the middle ear of birds suggests at least two differences from mammals in the ways the sound waves behave. Firstly, sound travels in the middle ear partly through gas in birds, whereas in mammals it enters a solid medium once past the eardrum. This may promote combined resonance inside the middle ear, which in birds cannot be strictly divided into left and right sides of the head. Secondly, airborne sound can simultaneously strike the avian eardrum from both outside (through the ear opening) and inside (through the middle ear) – something hardly possible in mammals.
In all birds, air enters the middle ear via the Eustachian tube, and then – to a far greater degree than in mammals – fills intricate diverticula in the cranium itself. This system of tympanic pneumaticity is as consistent in all living birds as is the lack of ear pinnae. The air passages in the auditory part of the skull are separate from and additional to the respiratory system of air sacs in the avian postcranial skeleton. They’re also separate from the anterior air sacs on the face.
The anatomy of tympanic air passages in birds has been known for more than two decades but nobody seems to have figured out the complex physics of how this airy labyrinth inside the cranium helps to determine the direction from which sound reaches the head. Owls have, it seems, evolved extra mechanisms of detecting the direction of sound. The ears of owls aren’t basically different from those of other birds but the avian resonant system may not work for the high-frequency sounds emitted by the prey of these birds.
Our line of thinking might possibly explain why owls and grouse don’t listen with the feathery ear pinnae that are available in prefabricated form in all birds. Instead, owls and other birds possessing paired feathery projections on their heads use these merely as head-crests, for display or its converse: camouflage by disruptive patterning.

We’ve already noted that both crocodilians and certain birds, with their opercula and pre-aural flaps, seem to have the anatomical and genetic capacity for the evolution of an ear pinna independent of mammalian evolution. We realise that we may be crossing the Bio-edge here into fanciful territory, but perhaps it’s not too outlandish to suggest the possible occurrence of ear pinnae in certain extinct archosaurs.
Please bear in mind that, although birds evolved from theropod dinosaurs, this doesn’t necessarily mean that dinosaurs shared the full system of internal resonant chambers in the skull. For example, dromaeosaurids, although in certain ways the dinosaurs most resembling birds, seem to have lacked the tympanic pneumaticity of the avian skull and – by inference – the hearing mechanism typical of birds.
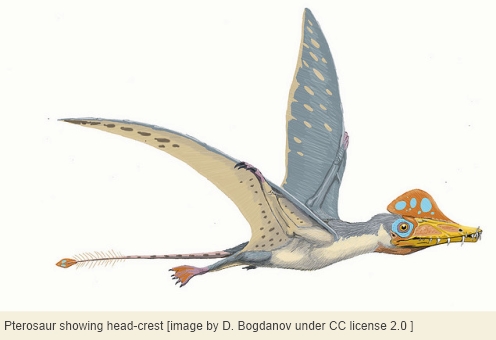
It’s easy to assume that all archosaurs lacked ear pinnae, but we wonder about certain duck-billed dinosaurs[15] and pterosaurs in particular. Please bear in mind that crocodilians and birds are known to have evolved a rudimentary external ear, dinosaurs are known to have evolved feathers, and pterosaurs are known to have evolved both their own fur-like insulation[16] and large head-crests[17] that seem to fly in the face of aerodynamic constraints. Given these facts, it no longer seems quite safe to assume that all archosaurs lacked ear pinnae.
Some of the more recently evolved duck-billed dinosaurs of the Cretaceous had elaborate bony tubes on the crown, connected to the respiratory passages. These are thought to have functioned as wind instruments during social calling. Since such sound-production has no parallel in any living reptile, maybe it’s not too far-fetched to suspect that hadrosaurs had particularly sophisticated hearing, perhaps including auricles convergent with those of mammals. After all, it’s also true that duck-billed dinosaurs were unusual in evolving a true grinding dentition, different from but analogous with that of herbivorous mammals. The fossil evidence for this dental mill not only shows a metabolic similarity to mammals but also implies more white noise while chewing than in other dinosaurs, perhaps compensated by enhanced reception of sound.
Because our explanation of the bird-mammal difference is only partial, it doesn’t rule out the possibility of a combination of pneumaticity and external ears in birds. So can palaeontologists continue to assume with confidence that certain extremely large-headed flightless birds, such as the terror birds[18] of the Americas, lacked both auricles and feathery ear pinnae?
Maybe we’re whistling in the wind. Maybe all of this will ring a bit hollow until some hard data turn up. But without the lateral thinking of a trip to the Bio-edge and the conjuring of a search-image for ear pinnae in dinosaurs, it doesn’t seem likely that palaeontologists would be lucky enough just to stumble across fossil evidence for or against such flimsy structures. And so we might miss some of the sweeter quavers that the bones and rocks could be whispering in our scientific ears.
***
All text and images appearing in this blog are subject to copyright, except those images explicitly stated to be in the public domain. You are not free to use any photographs, for any purpose, without receiving written permission from the copyright holder.
1 Owls in the genus Phodilus still need investigation, because they combine the facial disc of Tyto, which is known to have an auditory function, with small versions of the ‘ears’ of strigid owls.
2 Tytonidae: Tyto
3 i.e. by contraction of facial muscles
4 This asymmetry refers to the size and configuration of the ear opening and its vertical position on the skull, and in e.g. Tyto includes asymmetrical pre-aural flaps; the asymmetry is therefore both multidimensional and partly skeletal.
5 The alternative term, operculum, is too easily confused with various other body parts in vertebrates.
6 Asio otus
7 Living crocodilians possess a flap of skin and cartilage external to the ear opening, which cannot be called an auricle but is large enough to seal the ear during submersion.
8 Otus and related genera
9 Asio
10 Gallus gallus
11 Apterygiformes
12 i.e. facial vibrissae which, in the case of kiwi, are formed from bristle-like feathers
13 Steatornis
14 in the tympanic bullae
15 Hadrosauridae: Lambeosaurinae
16 i.e. pycnofibres
17 e.g. Pterosauria: Tapejaridae: Tupandactylus and Pterosauria: Thalassodromidae: Thalassodromeus
18 Phorusrhacidae, e.g. the Miocene Kelenken

